Corrosion protection of hydrogenation units
20
2024.8
Author
518
Read volume
With the development of the hydrogen energy industry, its upstream and downstream industrial structure has also changed accordingly. In particular, the preparation and transportation of energy have undergone huge reforms based on the original energy. One of the reasons is that hydrogenation units are prone to corrosion. If the previous machinery and equipment are used, not only will it not save costs, but it may cause hydrogen leakage or other more serious accidents during use, causing heavier losses.
Because there are several reasons for corrosion of hydrogenation units, we need to propose targeted protective measures:
1.& nbsp;Hydrogen damage:
A situation where hydrogen atoms diffuse into the metal or react with the metal to change the properties of the metal material, resulting in a decline in the overall properties of the metal material is called hydrogen damage. Hydrogen damage includes hydrogen embrittlement, high temperature hydrogen corrosion and hydrogen-induced exfoliation.
Protective measures:
(1) Strictly control the cooling and pressure reduction rates and should not be too large. Cooling rate:20~25℃/h, pressure reduction rate:1.0~1.5MPa/h. (Itcan well prevent hydrogen embrittlement and hydrogen-induced cracking). Overtemperature and overpressure of equipment are strictly prohibited.
(2) Control the content of impurity elements in the material, especially the hydrogen element content after acid cleaning, and carry out heat treatment after welding.
(3) Select appropriate hydrogen corrosion resistant materials, such as: austenitic stainless steel (which can be strengthened with nitrogen),low-alloy steel added with elements such as Cr, Mo, and V, precipitation-strengthened austenitic alloys and aluminum alloys (aluminum alloys have excellent resistance to environmental hydrogen embrittlement, but also show varying degrees of internal hydrogen embrittlement).

2.& nbsp;High-temperatureH2+ H2Scorrosion:
Metals will be corroded in anH2+ H2Senvironment at high temperatures above 204 ° C, manifested as uniform corrosion, hydrogen embrittlement and hydrogen corrosion.
Protective measures:
High temperatureH2S+ H2causes uniform corrosion, and the material selection of parts that are prone to corrosion must be reasonably designed.Generally, carbon steel can be used when temperatures exceed 250 ° C;chromium molybdenum steel (onlyH2exists) and or austenitic stainless steel(resistant toH2+ H2Scorrosion) can be used when temperatures exceed 250 ° C.

3.& nbsp;Polythionic acid corrosion:
Polythionic acid is usually formed by the reaction of sulfur-containing corrosion products with oxygen and water. Stress corrosion cracking will occur when the reaction products appear in sensitive austenitic stainless steel and other austenitic alloy process equipment.
Protective measures:
Select ultra-low carbon or stable austenitic stainless steel; try to eliminate or reduce residual stress caused by cold working and welding during manufacturing, and pay attention to processing into a structure with no stress concentration or as small as possible; Nitrogen protection, keep the equipment temperature at around150℃, and clean it.
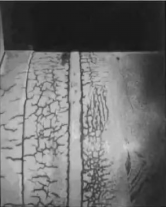
To sum up, we need to pay attention to:
1.& nbsp;Reasonable selection of materials, suitable materials can extend the service life of the machinery;
2.& nbsp;Control equipment temperature and cooling time to avoid high temperatures accelerating the corrosion reaction of materials;
3.& nbsp;Minimize impurities in materials and design structures reasonably.
(图源网络)
技术支持:郭洪华
编辑:刘康利


Online Selection

1V1 Customer Service

